OmicsArray™ 自身抗体检测服务(抗原芯片)
自身抗体与多种自身免疫性疾病和免疫系统失调有关。自身抗体检测服务主要用于自身抗体的检测,用抗原芯片分析针对一系列自身抗原的自身抗体图谱,可对自身免疫性疾病、过敏、肿瘤反应、接种疫苗和感染、器官移植等的免疫反应进行大规模分析,为疾病预警和诊断、治疗方法选择、疗效评估、预后分析等科学研究提供依据,也是发现具有临床价值的抗体标记、研究疾病发病机制的有力工具。
应用
OmicsArray™自身抗体检测芯片能用于以下研究,包括:
- 各种自身免疫性疾病,如:系统性红斑狼疮、类风湿性关节炎、糖尿病、混合结缔组织病、Sjøgren综合症、硬皮病、多肌炎、皮肌炎等等;
- 过敏性疾病;
- 传染性疾病;
- 脑神经失调或神经障碍;
- 病毒疫苗的开发及疫苗接种后对自身免疫反应的监测,如SARS-CoV-2、其他冠状病毒株、流感病毒和呼吸道合胞病毒(RSV)等。
- 癌症生物标志物和免疫调节治疗毒性监测;
- 器官移植效果评估;
- 药物筛选和临床试验。
产品优势
- 高多重性:与传统的ELISA法每孔检测1个抗体相比,自身抗原阵列每孔可检测>120个自身抗体;
- 高通量:每张玻片可同时处理16个样品,多张玻片可并行操作;
- 高灵活性:结合不同抗原(蛋白质、多肽、核酸等),检测不同类型和亚型的抗体;
- 小样本量:5 μL 血清或其他体液即可满足;
- 成本低:低于ELISA试剂盒1/10的价格;
- 高灵敏度:结果的动态范围在1-65000;
- 半定量:基于标准曲线的相对定量。
自身抗体检测
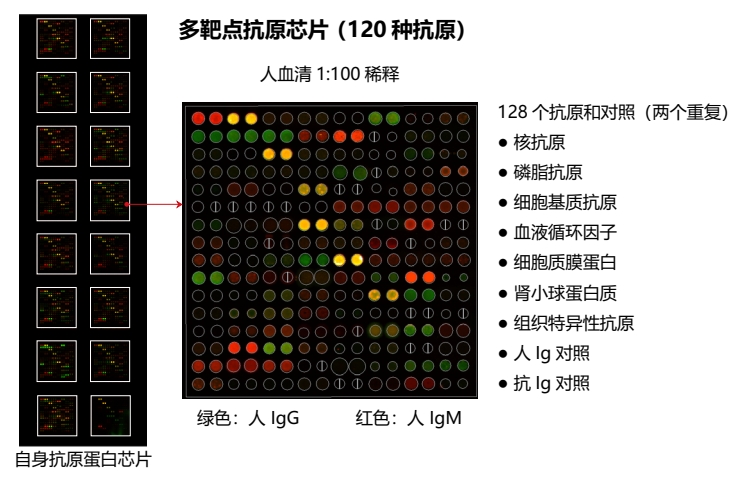
GeneCopoeia 的 OmicsArray™ 抗原微阵列包含多达 120 个纯化的抗原点,这些抗原点附着在玻璃载玻片上的硝酸纤维素滤片上。此外,还有 8 个点用于归一化。每个载玻片上有 16 个相同的阵列,因此可同时处理多达 15 个样本和 1 个阴性对照。
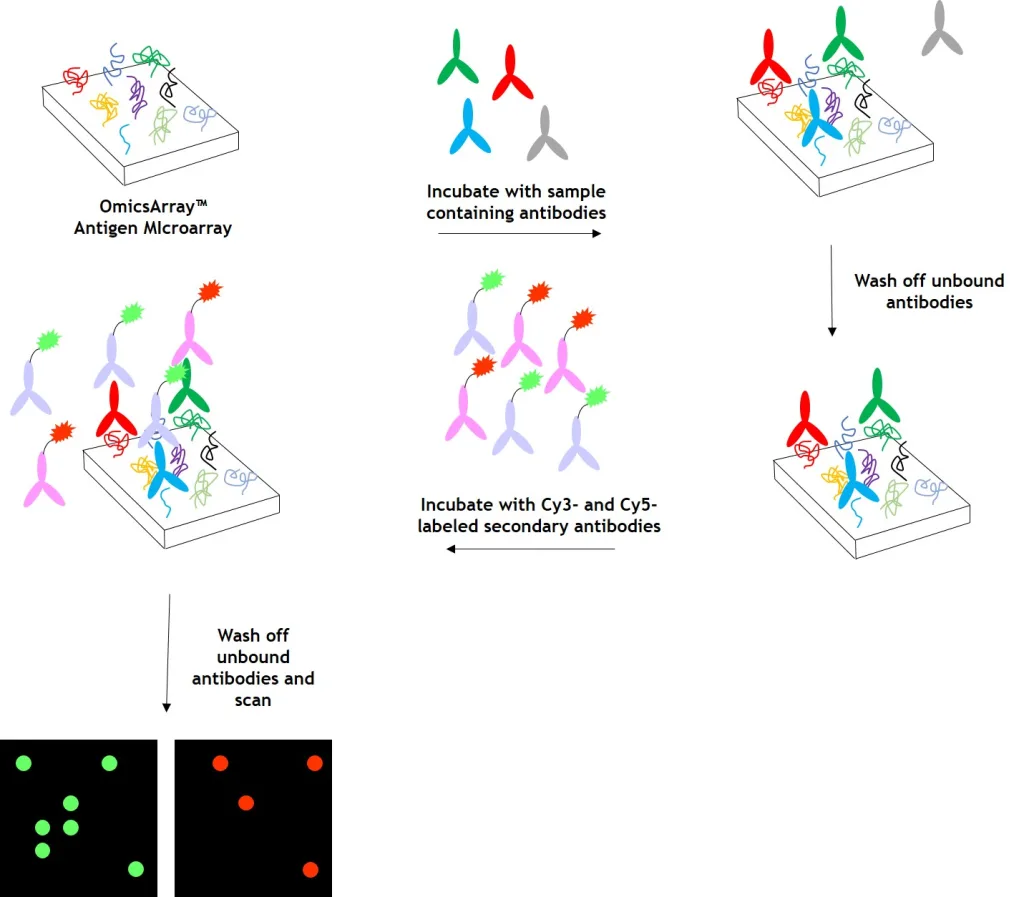
图2:使用 OmicsArray™ 抗原芯片对样本进行自身抗体检测。阵列与患者样本一起孵育,样本中的任何自身抗体都会与阵列上的同源抗原结合。
清洗阵列以去除未结合的自身抗体和其他蛋白质,然后与 Cy3 和 Cy5 标记的二抗共同孵育。双重标记策略旨在区分样本中存在的免疫球蛋白(Ig)亚型。例如,Cy3 标记的抗 IgG 二抗用于检测 IgG 自身抗体,Cy5 标记的抗 IgM 二抗用于检测 IgM 自身抗体。荧光团标记的二抗可用于检测 IgA、IgD、IgE、IgG 和 IgM 免疫球蛋白,以及 IgG 亚类 IgG1、IgG2、IgG3 和 IgG4。
洗涤去除未结合的二抗之后,使用微阵列扫描仪(如 GenePix® 4000B、InnoScan 710 或同等仪器)检测信号。然后使用 GenePix® Pro 7.0 或 Mapix 软件分析原始数据。
数据分析
GeneCopoeia的抗原芯片检测服务含简要数据分析。阵列扫描后,使用GenePix®7.0软件收集和分析原始数据。数据分析包括数据归一化、差异分析、聚类分析,以及按变化水平排列的每个抗原的热图。我们的分析按照以下步骤:
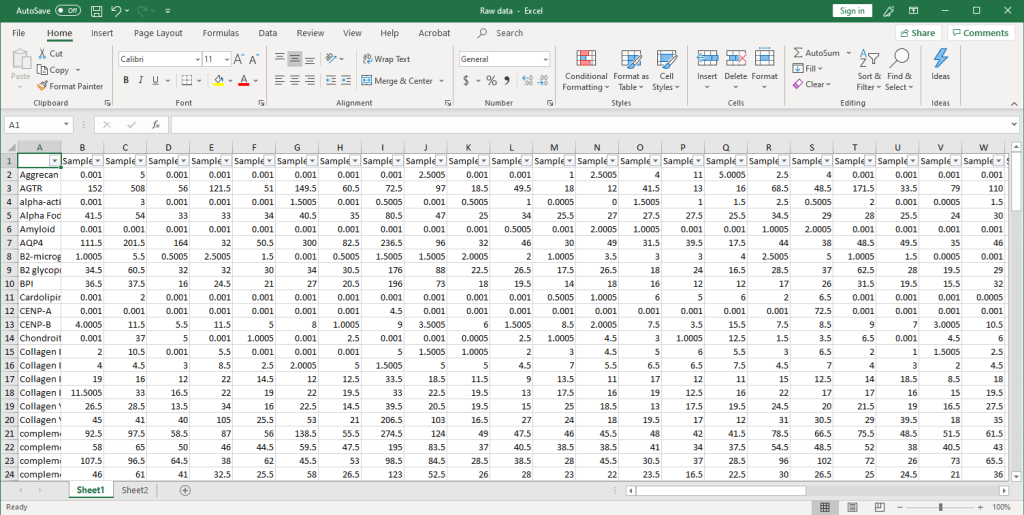
1. 收集原始数据。扫描后,在Excel文件中收集原始信号强度,如下所示(点击图片放大)。
2. 然后,将原始数据用参照点的值进行归一化,并将净信号强度(Net Signal Intensity,NSI)值以及信噪比(signal-to-noise ratios,SNRs)列在一个Excel文件中:
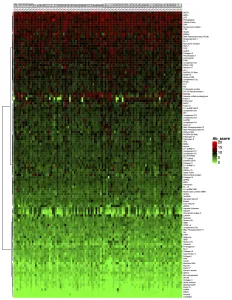
3. 标准分析的最后一步是确定“抗体评分”,即样品中给定抗体的相对富集程度。抗体评分以数字形式展示,并显示在热图中。得分越高(在热图上向红色移动)表明抗体-抗原相互作用越强,如下例所示:
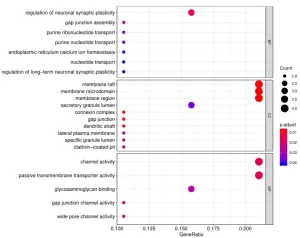
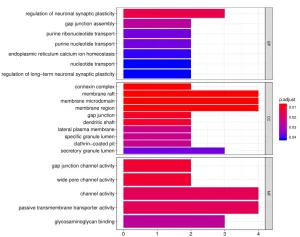
4. 除标准数据分析包外,客户还可以选择定制数据分析服务。其中一项服务是使用基因本体(Gene Ontology, GO)分析对阵列上的阳性抗原进行分类,该分析基于已知的生物功能对基因和蛋白质进行分类。分子功能GO分析的读数示例如下所示:
此外,生物过程的GO分析如下示例所示:
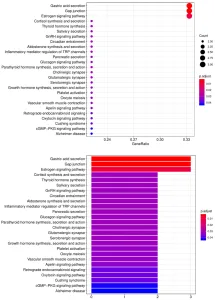
5. 另一个广泛用于生物信息分析的工具是KEGG(Kyoto Encyclopedia for Genes and Genomes)通路分析,我们使用该工具根据已定义的生物通路对阳性抗体-抗原相互作用进行分组,如下所示:
客户定制
自身抗体与多种自身免疫性疾病和免疫系统失调有关。自身抗体检测服务主要用于自身抗体的检测,用抗原芯片分析针对一系列自身抗原的自身抗体图谱,可对自身免疫性疾病、过敏、肿瘤反应、接种疫苗和感染、器官移植等的免疫反应进行大规模分析,为疾病预警和诊断、治疗方法选择、疗效评估、预后分析等提供依据,也是发现具有临床价值的抗体标记、研究疾病发病机制的有力工具。
Genecopoeia提供用于转化医学研究的,针对自身抗体检测的芯片服务。除了预制的自身抗体检测芯片,还可提供定制服务。如需定制自身抗体检测芯片,请点击此处,填写服务表格。
抗原芯片
| 货号 | 描述 | 应用 |
| PA001 | 自免病相关的自身抗体检测,120种抗原 | 能够高效地检测与许多疾病相关的自身抗体,包括类风湿关节炎、肌肉营养不良、系统性红斑狼疮和 I 型糖尿病。 |
| PA002 | 神经免疫相关的自身抗体检测,120种抗原 | 能够高效地检测与神经系统疾病相关的自身抗体。 |
| PA003 | 癌症相关的自身抗体检测,120种抗原 | 能够高效地检测与癌症和癌症检查点免疫疗法相关的自身抗体。 |
| PA004 | 抗磷脂综合症和中性粒细胞细胞外捕获物相关的天然形式蛋白自身抗体检测,120种抗原 | 能够检测与系统性自身免疫性疾病有关的抗体,包括抗磷脂综合症(APS)、类风湿性关节炎(RA)和全身性红斑狼疮(SLE)。 |
| PA005 | 抗磷脂综合症和中性粒细胞细胞外捕获物相关的瓜氨酸化蛋白自身抗体检测,120种抗原 | 能够检测与系统性自身免疫性疾病有关的抗体,包括抗磷脂综合症(APS)、类风湿性关节炎(RA)和全身性红斑狼疮(SLE)。 |
| PA006 | 过敏原的自身抗体检测,70种抗原 | 能够高效地检测与常见过敏原相关的抗体。 |
| PA007 | 细菌表面抗原芯片检测,14种抗原 | 能够同时检测针对人类14种潜在致病细菌菌株表面抗原的抗体 |
| PA008 | 抗可溶性抗原和抗核抗体检测,32种抗原 | 可高效检测与系统性自身免疫性风湿病(如系统性红斑狼疮(SLE)、系统性硬化症(SSc)、Sjögren综合征(SS)和混合性结缔组织病(MCTD)等)相关的自身抗体。 |
| PA010 | 感染和疫苗接种相关的自身抗体检测,120种抗原 | 能够有效地检测与常见病毒和疫苗诱导的自身免疫相关的抗体。 |
| PA011 | 干扰素、感染和自身免疫性相关自身免疫抗体检测,64种抗原 | 能够有效地检测与病毒感染和自身免疫性疾病相关的抗体。 |
| PA012 | 冠状病毒感染相关的自身抗体检测,120种抗原 | 可用于定量检测COVID-19患者针对多种抗原的各种抗体亚型(例如IgG、IgM、IgA),并用于评估患者对病毒感染和疫苗接种的免疫反应。 |
| PA013 | COVID-19冠状病毒变异抗原的自身抗体检测,58种抗原 | 在人类血清/血浆中检测SARS-CoV-2(野生株和变异株)抗体(IgG、IgM、IgA)、其他冠状病毒抗体和其他常见呼吸道病毒抗体 |
| PA014 | 蛋白质组学抗原的自身抗体检测,~16000种抗原 | 能够高效检测样品中蛋白质组层次的蛋白相互作用,用于检测患者体液中与自身免疫疾病相关的抗体以及发现潜在的新型自身抗体。 |
| PA015 | 神经节苷脂和磷脂抗原的自身抗体检测,21种抗原 | 能够检测神经系统自身免疫性疾病的自身抗体,包括格林-巴利综合征、脊髓炎、布朗-切卡德综合症和米勒-费雪综合症。 |
| PA016 | 自身免疫性心肌炎和肌炎抗原阵列,120种抗原 | 能够检测与自身免疫性心肌炎、肌炎和其他自身免疫性疾病相关的自身抗体。 |
| PA017 | 重症肌无力的自身抗体检测,120种抗原 | 能够检测与重症肌无力相关的自身抗体。 |
服务流程
点击下载 服务指南 
样本要求
1. 适用于自身抗原芯片检测的常见样品
血浆、血清、淋巴、尿液、间质液、渗出液、细胞溶解液、分泌液等体液均可用于自身抗原芯片检测分析。
2. 样品的收集方法
a. 血清:采用不含抗凝剂的采血管采集全血,4℃放置30-45min后,4℃ 3000rpm离心10min,取上清,存于-80℃冰箱;
b. 血浆:采用抗凝剂的采血管采集全血,4℃放置30-45min后,4℃ 3000rpm离心10min,取上清,存于-80℃冰箱;
c. 尿液:收集不添加稳定剂的尿液样本后,4℃ 10000rpm离心1min或5000rpm离心2min,取上清,在干冰/甲醇浴中快速冷冻,储存在-80℃备用。
3. 样本量
为保证实验的顺利进行,体液样本的样本量请≥50 ul。
4. 样本寄送要求
a. 快递发出后请于当天将快递单号或专业化冷链物流托运单号及时告知乙方;
b. 收货后乙方会对样品到达状态拍照,若样本送达状态不佳,可能需重新寄送;
c. 体液样本请优先保存于干冰中寄出,干冰用量需足够以确保寄到时仍有干冰剩余;
d. 不能使用干冰的情况下请使用冰袋,冰袋数量需足够以确保寄到使样本温度低于4℃;
报告形式
服务的实验结果将通过邮件形式提供相应的实验数据资料及报告文件。基本包括:
- 蛋白芯片扫描图;
- 杂交信号强度值;
- 差异分析;
- 聚类分析等。
在自身免疫性疾病发生、发展的过程中,引起自身免疫应答的自身组织成分被称为自身抗原,包括隐藏的自身抗原(在胚胎期从未与自身淋巴细胞接触过,机体不能识别为自身物质)和修饰的自身抗原(在感染、药物、烧伤、电离辐射等因素影响下,自身组织的构象发生改变,成为自身抗原)。机体产生自身抗原时,由于免疫系统将其识别为异己部分,会产生对应的自身抗体进行抵抗。
自身抗原芯片,是指以蛋白质分子作为配基,将其有序地固定在固相载体的表面形成微阵列,用标记了荧光的蛋白质或其他它分子与之作用,洗去未结合的成分,经荧光扫描等检测方式测定芯片上各点的荧光强度,来分析蛋白之间或蛋白与其它分子之间的相互作用关系。
OmicsArray™抗原芯片上的抗原,全部是来自于文献报道的自身抗原。对于每种不同类型的抗原芯片,在您确定签署相应服务协议后,我们会给您提供全部的抗原及相关信息,在您使用自身抗原芯片的检测结果发表文章之前,请勿对第三方展示此信息。
这些自身抗原或自身抗体在疾病表型显现的前期往往已经产生,并且表达量通常随着病情的演化呈现出一定的趋势,是一类重要的疾病生物标志物。越来越多科研人员将目光定格在自身抗体相关标志物的研究上,力求在自身抗体层面寻找生物标志物,建立诊断、预后、药效评估的多参数模型。 为了满足疾病诊断,预后判断等多方面的广泛而长期的需求,疾病生物标志物的发现研究不可或缺。基于疾病相关自身抗体的标志物因在多种疾病中广泛存在,且可通过血液样本而方便地实现检测而备受关注。
请在实验之前与售前进行充分沟通,说明实验目的,样本情况,以明确我方是否能满足您的需求以及确定实验方案。
- 由于每张芯片上有16个block,其中一个用来做PBS对照,剩下15个用来孵育样本。所以客户所送样本的总数量最好为15的倍数,这样可以充分保证芯片的使用效率,避免让客户分担相应的损失成本。
- 理论上每个不同的检测组最低需要3个样本才能满足统计学上重复性的要求。在此基础上,样本越多,其结果对检测组的代表性越高。为了保证结果的可靠性,推荐不同检测组样本量≥30。
抗原芯片采用荧光检测,其灵敏度要比ELISA(比色法)、Western(化学发光法)更加灵敏和稳定。片内和片间的技术重复相关系数R2均可以达到0.9以上。动态检测范围在1-65000。动态范围越大,说明所能检测的信号层次就越多。
如果您需要定制芯片,我们会先与您就以下问题进行充分沟通:
- 抗原的知识产权情况(是来自于已公开发表的论文还是已受专利保护等),我们仅能提供不涉及专利保护的抗原定制检测;
- 抗原的基本信息(是全蛋白还是肽段,分子量,结构域,是否为膜蛋白等);
- 您是否能提供满足定制芯片要求的抗原;
- 若需要我方提供待检抗原,相应费用及实验周期需另外核算。
客户可以选择是否需进行预实验,若进行预实验,客户可提供15例样本(需符合样本要求),若您后续继续签署服务,且预实验的数据可用,再记入正常样品收费。
a. 这个差异也许与检测手段不同相关,芯片用的蛋白为非变性蛋白,和WB有所差别;芯片的杂交体系为抗原捕获抗体,与WB的抗原转膜后与抗体孵育也稍有差别,因此也许会造成部分差异;
b. 需要客户提供WB验证的结果图,以便我们更好的分析分析客户验证的指标在芯片上的图像和数据情况;
目前应用自身抗原芯片发表的研究类文章已经非常多。我们的自身抗原芯片已在全世界近百家医疗科研和医药企业得到广泛应用,已独立或和用户合作发表了几十篇论文。其中高影响因子的论文含数十篇。蛋白芯片技术可以极大提高科学研究的效率,更能够获得较为全面的数据,以在研究中得到创新的结果和结论,是科学论文发表的加分项。